Address:Room 906, building 6, SIIC Center, No.195 Hong Kong East Road, Laoshan District, Qingdao city, Shandong Province, China
E-mail:info@deltachem.net

The oxidation process of polymer is a chain reaction of free radical type. Plastic antioxidants can catch the active free radicals, and generate inactive free radicals, or decompose the hydroperoxides that generated in the oxidation process, so as to terminate the chain reaction and delay the oxidation process of the polymer, so that the polymer can be processed successfully and its service life can be prolonged. Among the antioxidants for long-term thermo oxidative stability protection of polymers, hindered phenolic antioxidants are the extremely important antioxidants which can be used alone or in combination with auxiliary antioxidants. The combination effect brings better long-term and high-temperature thermal oxygen stabilization effect than using phenolic antioxidant alone.
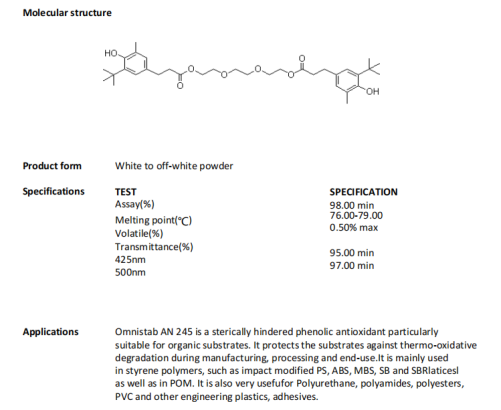
The reduction of steric hindrance effect of asymmetric hindered phenol antioxidant (AN245) makes it easy to realize the substitution of nitro group on its hydroxyl group of phenol. And the white solid generalized in this process the M-nitro compound solves the problem that symmetric hindered phenol m-nitro oxidation turns into coloring product.
Synergistic use of asymmetric hindered phenol antioxidant(AN245) and thioester antioxidant compared with the synergistic use of symmetric hindered phenol antioxidant (AN1010) and thioester antioxidant. The result shows that when AN245 and AN1010 were used as main antioxidants alone, the thermal oxygen stability of the two was almost the same. However, when the main antioxidant and thioester auxiliary antioxidant are used together, the thermal oxygen stabilization effect of asymmetric hindered phenol antioxidant(AN245) is obviously better than its of symmetric hindered phenol antioxidant (AN1010) coupled with the thioester auxiliary antioxidant.
The data further verified that there was intermolecular hydrogen bond association between the phenol hydroxyl group of asymmetric hindered phenol antioxidant and the ester bond of thioester, and the combination was relatively close in the polymer system, which was conducive to the effective decomposition of hydroperoxide. For symmetric hindered phenol antioxidants, due to the existence of tert butyl groups in the two ortho positions, a large steric hindrance effect is produced, which hinders the formation of molecular hydrogen bond between phenol hydroxyl group and thioester bond. The hydroperoxide produced by hydrogen transfer of the main antioxidant can only react with thioester indirectly and randomly, so the decomposition efficiency of hydroperoxide is not high. Therefore, the synergistic effect of asymmetric hindered phenol antioxidant and thioester is significantly higher than that of symmetric hindered phenol antioxidant and thioester.
DELTACHEM(QINGDAO)CO.LTD focused on the R & D, production and sales of polymer additives, you are welcomed to consult more subject about the polymer additive. Please contact our sales for further information.